A Milestone for Pompe Families This September
September is Newborn Screening Awareness Month, a time to celebrate the programs that ensure every baby has a healthy start. This year, there’s especially good news for families affected by Pompe disease: on August 18, 2025, Texas has officially added Pompe disease to its newborn screening panel, marking a major milestone in the fight for early detection.
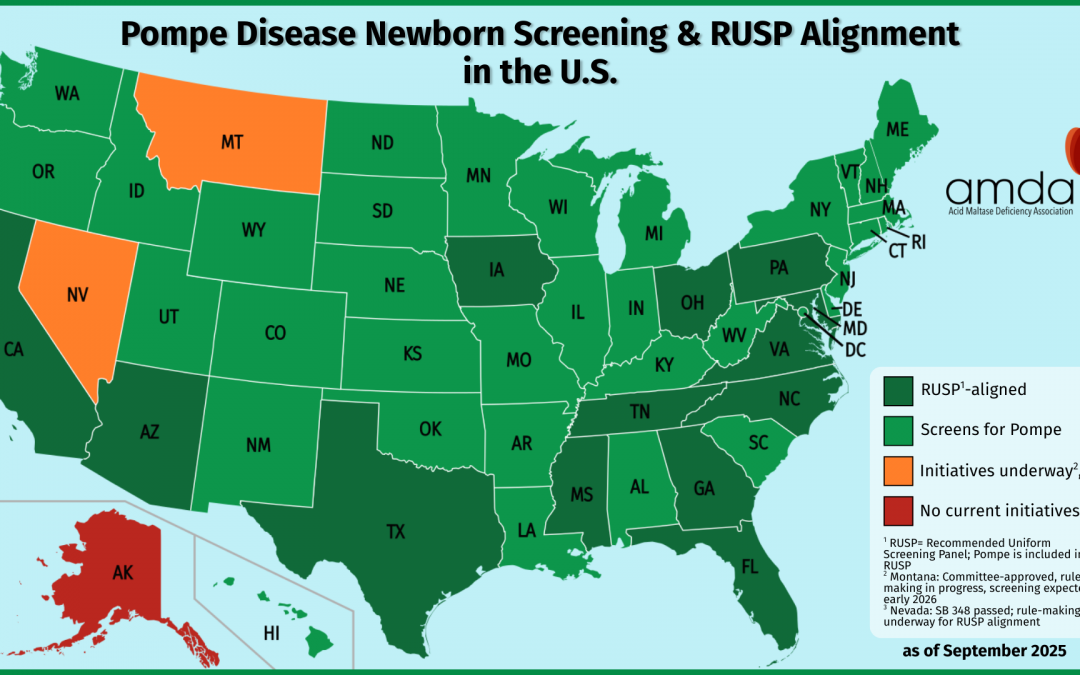
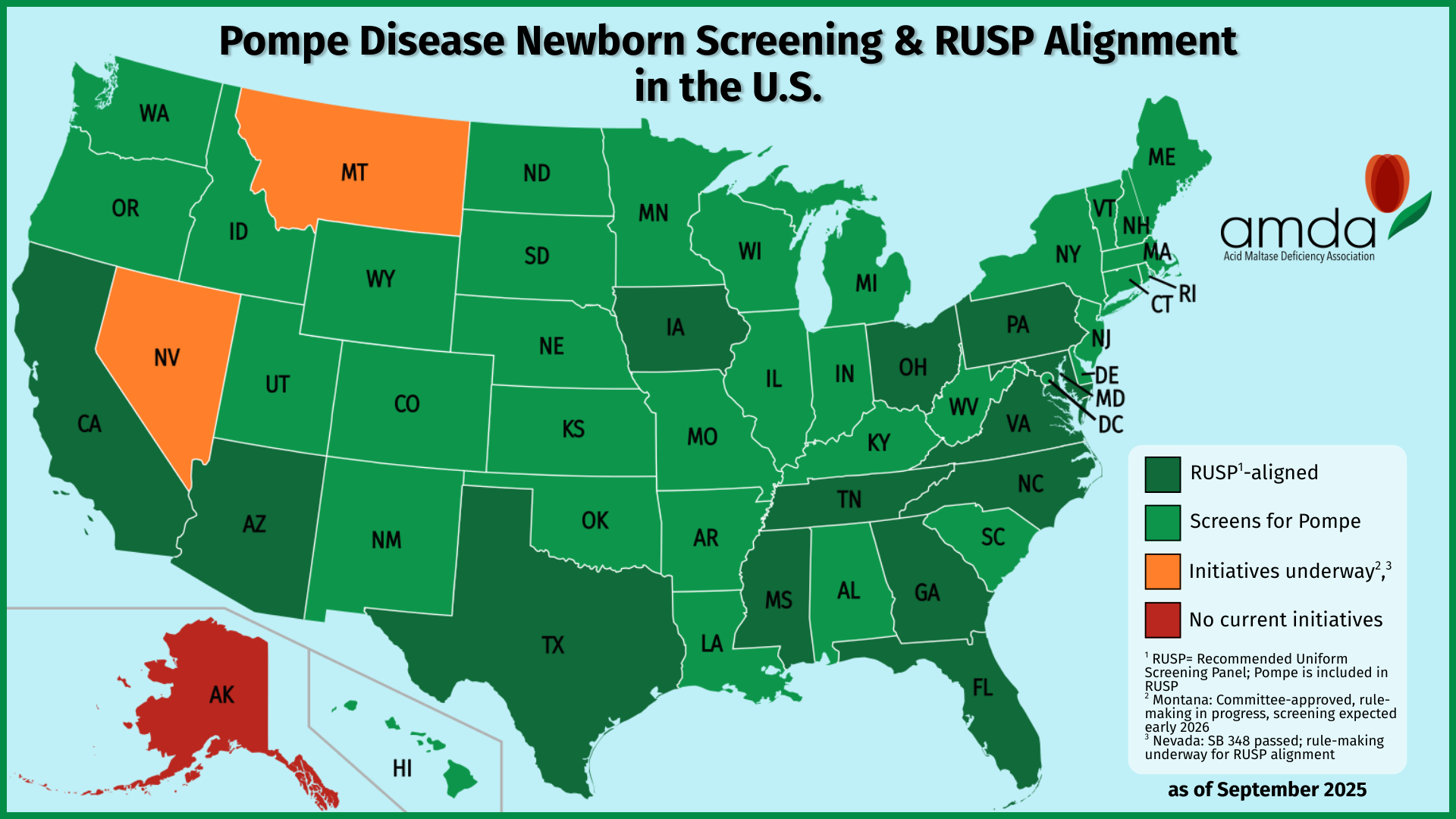
At the AMDA, we wanted to celebrate by taking a snapshot of the nation: which states currently screen for Pompe disease? As we dug into the data, we discovered some truly encouraging progress—47 states plus the District of Columbia now screen for Pompe, and all but one (Alaska) have plans to implement screening by the end of 2026.
But while this is a moment to celebrate, it also highlights the next frontier in newborn screening equity: only 14 states have RUSP alignment legislation, which means that in many states, babies’ access to new screenings can still depend on geography and bureaucratic timelines.
In this post, we’ll share our national Pompe screening map, explore why RUSP alignment matters, and provide context for how families, advocates, and policymakers can work together to ensure all newborns have access to the life-saving screenings they need.
Who Screens for Pompe Today?
Map By Heitordp – File:Blank USA, w territories 2.svg, modified to remove territories and move Alaska slightly, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=90875180
To celebrate Texas joining the ranks of states screening for Pompe disease, we created a current national map showing which states screen newborns for this condition—and how they align with federal RUSP guidance. The visual makes it immediately clear: screening for Pompe is nearly universal, and families across the country are benefitting from earlier detection and access to treatment.
Here’s what the map shows:
- Dark green states: These states not only screen for Pompe but also have RUSP alignment legislation, ensuring future conditions are reviewed and adopted on a set timeline. There are currently 14 states in this category.
- Light green states: These states screen for Pompe but do not yet have full RUSP alignment legislation.
- Orange states:
- Montana is in the process of adding Pompe through its state newborn screening advisory committee.
- Nevada is implementing Pompe screening following RUSP alignment legislation (SB348), signed by the governor on June 6, 2025; it is marked orange instead of dark green because it does not currently screen for Pompe.
- Red states: States like Alaska that do not have any current plans to add Pompe to their newborn screening panels by the end of 2026.
Currently, 47 states plus the District of Columbia screen for Pompe disease.
This snapshot is not just a picture—it’s a story of progress. In 2015, Pompe disease was first added to the RUSP, and now, a decade later, nearly every U.S. newborn has the chance to be screened. Each color-coded state represents a family whose child might now receive earlier diagnosis and life-saving treatment.
While the map highlights tremendous strides, it also underscores why policy alignment matters: states with RUSP alignment legislation are positioned to implement new screenings more quickly and consistently, whereas others may experience delays. In the next sections, we’ll explore why RUSP alignment is critical for ensuring equitable newborn screening for all conditions.
From Federal Recommendations to Local Reality
The Recommended Uniform Screening Panel (RUSP) is the federal guideline for which conditions every newborn should be screened for. It’s created through a rigorous, evidence-based process by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) and approved by the HHS Secretary. The RUSP is designed to ensure that screening programs are safe, effective, and beneficial to babies and families.
However, it’s important to understand that the RUSP is guidance, not a mandate. Each state controls its own newborn screening program, deciding which conditions to add and when. That’s why, even though Pompe was added to the RUSP in 2015, states adopted screening on different timelines. The map you just saw illustrates this reality:
- Some states have full RUSP alignment legislation (dark green), so they can implement new RUSP conditions quickly.
- Others screen Pompe but don’t have alignment laws (light green), meaning future additions may take longer.
- A few states are in transition (orange), like Montana and Nevada, showing how different mechanisms—advisory committee review versus RUSP-alignment legislation—affect adoption speed.
- And states like Alaska (red) highlight that babies’ access to screening still depends on where they are born.
Using Pompe as an example, we can see why RUSP matters: early identification allows babies to start treatment sooner, which can prevent or minimize serious complications. But without state adoption and alignment, that benefit isn’t guaranteed for every family.
In the next section, we’ll explore why state-by-state adoption can lag, what causes those delays, and how RUSP alignment legislation helps ensure equitable newborn screening across the country.
Closing the Gaps: Beyond Pompe
Even after a condition is added to the RUSP, implementing newborn screening in every state isn’t instantaneous. On average, it takes five to six years for states to adopt a new condition after federal recommendation. This lag happens for several reasons:
- Laboratory readiness: States need time to validate tests, train staff, and upgrade equipment.
- Funding and resources: New screenings often require additional staffing, reagents, and follow-up systems.
- Legislative or regulatory processes: Some states need approval from advisory committees, health departments, or lawmakers before adding a condition.
- Programmatic priorities: States may focus on other screenings or public health needs before expanding their panels.
Pompe disease illustrates these challenges. Recommended for RUSP inclusion in 2015, it has taken nearly a decade for screening to reach almost every U.S. state and D.C. Some states adopted quickly, while others relied on advisory committees or legislation to guide their timeline.
This state-by-state variation has real consequences. A baby born in a state without screening may experience delayed diagnosis, potentially missing the critical window for early treatment. In contrast, babies born in states with RUSP alignment laws benefit from more consistent and timely access.
The good news is that awareness of theses gaps has grown, leading to policy innovations like RUSP alignment legislation, which we’ll explore in the next section. These laws aim to shorten adoption delays and ensure that every newborn, no matter where they are born, can access the same life-saving screenings.
Why 14 States Lead the Way
While nearly all states now screen for Pompe disease, the timeline for adding new conditions can still vary dramatically without formal policy mechanisms. That’s where RUSP alignment legislation comes in.
What is RUSP alignment legislation?
These laws require states to review and adopt new RUSP conditions on a set schedule, rather than waiting for advisory committees, budget cycles, or other ad hoc processes. They often include funding and resources for laboratory readiness, follow-up, and staff training, helping states implement screenings more quickly and efficiently.
Currently, 14 states—including 13 that have implemented Pompe screening and Nevada, which has legislation but is still rolling out screening—have RUSP alignment laws. In contrast, most states that screen Pompe do not yet have alignment legislation, and Alaska remains the only state without plans to add Pompe by the end of 2026.
Using Pompe as an example, we can see the practical impact: states with alignment laws were able to act quickly once the RUSP recommended it, while states without such legislation may still experience delays.
Why it matters:
RUSP alignment legislation doesn’t just speed up adoption; it creates equity. All babies, regardless of where they are born, deserve the same opportunity for early detection and treatment. Highlighting these 14 states shows what progess looks like when policy and science align, and why broader adoption of such legislation is the next step in strengthening newborn screening nationwide.
Toward True Equity in Newborn Screening
The progress in Pompe disease screening is a story worth celebrating. In just a decade since its addition to the RUSP in 2015, nearly every U.S. state and the District of Columbia will be screening newborns for Pompe by the end of 2026. Texas’ launch in August 2025, Nevada’s passage of RUSP alignment legislation in June 2025, and Montana’s implementation plan to add Pompe to their panel at the begining of 2026 is a reminder of how far we’ve come—and how coordinated policy and advocacy can drive meaningful change.
Yet, as the map illustrates, only 14 states have RUSP alignment legislation. That means in many parts of the country, babies’ access to new screenings can still depend on geography and legislative timelines. Achieving nationwide equity in newborn screening requires continued awareness, advocacy, and action.
How You Can Help
Families, advocates, and healthcare professionals all play a role in pushing for RUSP alignment legislation in their states. Here are a few ways to get involved:
- Contact your state legislators
- Reach out to your state representatives and senators to ask them to support bills that align newborn screening with RUSP recommendations.
- Share your story or explain why early detection saves lives and reduces long-term healthcare costs.
- Engage with state newborn screening advisory committees
- Attend meetings (many are public) or submit comments when new conditions are under review.
- Advocate for the adoption of RUSP-aligned policies and funding for laboratory readiness.
- Partner with advocacy organizations like the EveryLife Foundation
- EveryLife provides resources, guidance, and legislative support to help advocates push for state-level newborn screening policies.
- Collaborate on campaigns, social media efforts, or outreach to state legislators to promote RUSP alignment legislation.
- Use EveryLife’s tools and templates to share personal stories, explain the benefits of early detection, and make the case for timely adoption of new screenings.
- Educate your community
- Raise awareness about the importance of RUSP alignment legislation through social media, local events, or newsletters.
- Highlight success stories like Texas and other RUSP-aligned states to show the impact of timely adoption.
By taking these steps, you can help ensure that all newborns, no matter where they are born, have access to the life-saving screenings they need. Progress is possible, and each voice makes a difference.
Frequently Asked Questions (FAQs)
What does Newborn Screening entail?
Newborn screening includes three main tests: a blood test, a hearing test, and a pulse oximetry test.
- Blood test (Newborn Screening): Performed by a nurse, midwife, or trained staff member, it involves a small blood sample taken from the baby’s heel. The sample is collected on special filter paper and sent to a state lab for analysis.
- Pulse oximetry (pulse ox test): This non-invasive test measures oxygen levels in the blood and can detect heart conditions called Critical Congenital Heart Disease. A painless sensor is placed on the baby’s skin for about 10 minutes, usually before leaving the hospital.
- Hearing test: Can be done using either an auto acoustic emissions test or an auditory brainstem response test, or both. These quick, safe, and comfortable tests can even be done while the baby is asleep.
Watch this excellent video from Expecting Health What is Newborn Screening?
When are Newborn Screening tests typically done?
Blood, hearing, and pulse ox tests are typically performed within the first 24-48 hours after birth.
- Blood taken too early (before 24 hours) may miss some conditions.
- Waiting longer than 48 hours can delay crucial care.
Do I need to request Newborn Screening for my baby?
If you give birth in a hospital, newborn screening is routinely performed as part of standard care. If you give birth outside of a hospital (birthing center, home birth), coordinate with your healthcare provider beforehand to ensure your baby is screened at the appropriate time.
What is the RUSP and why is it important?
The Recommended Uniform Screening Panel (RUSP) is a federal guideline listing conditions that all newborns should ideally be screened for. It ensures screenings are safe, effective, and beneficial.
States control their own screening programs, so RUSP alignment legislation is critical for making sure new screenings are adopted quickly and equitably.
How many states currently test for Pompe disease?
47 states and the District of Columbia currently screen for Pompe disease.
- Montana is planning to add Pompe in early 2026.
- Nevada is planning to add Pompe in late 2026.
- Alaska currently has no confirmed plans to add Pompe screening.
Why isn't Pompe screening available in all U.S. states yet?
Newborn screening programs are run by each state, which is why availability can vary. Historically, newborn screening began at the state level, and as programs evolved, each state made independent decisions about which conditions to screen for, when to expand the panel, and how to fund and implement testing.
Other factors influencing implementation include:
- Advisory committee review and approval
- Laboratory readiness and staff training
- Legislative or regulatory processes
- Progammatic priorities and funding
RUSP aligment legislation helps states adopt new screenings more quickly and consistently, reducing these delays.
What happens if a baby tests positive for Pompe disease?
A positive newborn screen is not a diagnosis. Confirmatory testing is required, which may include:
- Repeat screening tests
- Referral to a metabolic or genetic specialist
If Pompe disease is confirmed, treatment—typically Enzyme Replacement Therapy (ERT)—will be started as soon as possible.
Why is it important to start treatment for Pompe disease as soon as possible?
Pompe disease is progressive, meaning symptoms worsen over time. Early treamtent is critical to prevent irreversible damage to muscles and organs, especially the heart.
- Babies with infantile-onset Pompe disease (IOPD) may not survive the first year without treatment.
- Starting ERT before six months of age dramatically improves survival and quality of life.
Can newborn screening detect late-onset Pompe disease?
Yes. Screening can identify babies who carry genetic variants associated with Pompe disease, including those that may lead to late-onset forms.
- These babies may not show symptoms at birth, but early identification allows families and doctors to monitor closely and begin treatment promptly if symptoms develop.
Are there any risks associated with newborn screening for Pompe disease?
Newborn screening is low risk. The blood test requires only a small heel-prick sample, and results guide further evaulation if needed.
- A positive screen does not mean the baby definitely has the condition; it indicates the need for confirmatory testing.
- Benefits of early detection far outweigh the minimal risks associated with the screening itself.
How can families prepare if their baby screens positive for Pompe?
- Connect early with a metabolic or genetic specialist. Our patient advocate, Marsha Zimmerman, can help families find Pompe care in their area.
- Learn about treatment options, including ERT.
- Join Pompe disease social media support groups to connect with other families, share experiences, and access resources.
- Keep copies of screening and follow-up results for easy reference.
What happens if my baby is born in a state that does not screen for Pompe?
If Pompe screening is not available in your state, you can:
- Ask your healthcare provider about reflex or commercial testing options.
- Advocate with your state newborn screening program to adopt Pompe screening.
- Connect with advocacy organizations like EveryLife Foundation for guidance and resources.
How can parents advocate for expanded newborn screening?
Families can take action by:
- Contacting your state legislators
- Ask your representatives and senators to support bills that align newborn screening with RUSP recommendations.
- Share your story or explain why early detection saves lives and reduces long-term heathcare costs.
- Engaging with state newborn screening advisory committees
- Attend meetings (many are public) or submit comments when new conditions are under review.
- Advocate for the adoption of RUSP-aligned policies and funding for laboratory readiness.
- Partnering with advocacy organizations like the EveryLife Foundation
- EverLife provides resources, guideance, and legislative support to help advocates push for state-level newborn screening policies.
- Collaborate on campaigns, social media effots, or outreach to state legislators to promote RUSP alignment legislation.
- Use EverLife’s tools and templates to share personal stories, explain the benefits of early detection, and make the case for timely adoption of new screenings.
- Educating your community
- Raise awareness about the importance of RUSP alignment legislation through social media, local events, or newsletters.
- Highlight success stories like Texas and other RUSP-aligned states to show the impact of timely adoption.
By taking these steps, you can help ensure that all newborns, no matter where they are born, have access to the life-saving screenings they need. Progress is possible, and each voice makes a difference.