Aug 6, 2021 | NEWS
PARIS – August 6, 2021 – The U.S. Food and Drug Administration (FDA) has approved Nexviazyme® (avalglucosidase alfa-ngpt) for the treatment of patients one year of age and older with late-onset Pompe disease, a progressive and debilitating muscle disorder that...
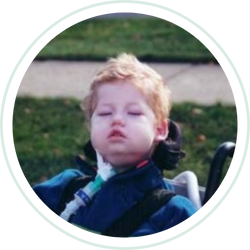
Aug 5, 2021 | PATIENTS
A year ago today [April 29, 2004] our son, John, passed away from Pompe Disease. He was four years old. Most of you will not recognize his name, but you all should know who he is. John was patient 101 – the first person to receive the CHO Enzyme Replacement Therapy...

Aug 5, 2021 | PATIENTS
A Mothers Anguish April 6, 2002, was the saddest day of my life. Senselessly, while in the care of an inept medical system, my wonderful daughter passed away. Her name was Patty. Everyone, especially the medical profession, should be aware of the potential for this to...

Aug 5, 2021 | PATIENTS
This is the story of me from before I was diagnosed with Pompe’s disease. I hope this helps to enlighten the medical community as well as other Pompe patients. Back in 1965 my Mom was told that I would be born on December 2nd. This date came and went and the...
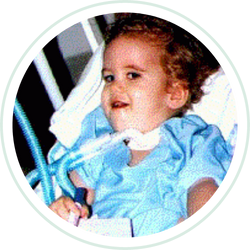
Aug 5, 2021 | PATIENTS
Our son Luke, the youngest of four children, was diagnosed with Pompe’s Disease at 5 months of age. He had a very normal birth and development but was a floppy baby. Luke was first hospitalized at 6 months of age with RSV virus and pneumonia after which he was...




